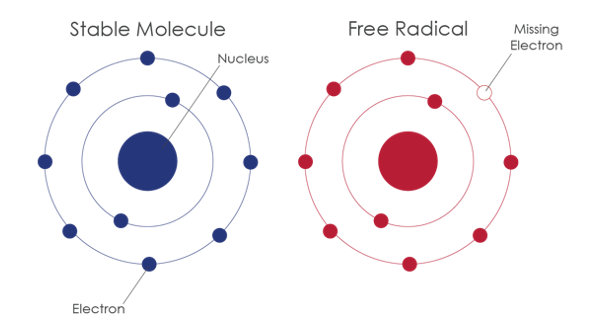
Free radicals
The human body is composed of many different types of cells. Cells are composed of many different types of
molecules. Molecules consist of one or more atoms of one or more elements joined by chemical bonds. Atoms
consist of a nucleus, neutrons,protons and electrons. The number of protons (positively charged particles) in the
atoms nucleus determines the number of electrons (negatively charged particles) surrounding the atom. Electrons
are involved in chemical reactions and are the substance that bonds atoms together. But on nature situation our
body will produce unpaired electrons called free radicals, these radicals are cause from complicated chemical
reactions, metabolism, respiration and cells wastes from our body.
On the normal situation our body automatically forms free radicals the reason is we need oxygen to maintain our
life everyday so finally we aging and dead. But if we have bad modern life style will also leads to excess of free
radicals. These several unpaired free radicals they are always steal other electrons to become stable. Out in the
world, this is a normal process, but in the body, it can result in unnecessary damage like quick aging, tissue damage,
cancers and possibly some diseases.
Although oxidation reactions are crucial for life, they can also be damaging; hence, plants and animals maintain
complex systems of multiple types of antioxidants, such as glutathione, vitamin C, and vitamin E as well as enzymes
such as catalase, superoxide dismutase and various peroxidases. Low levels of antioxidants, or inhibition of the
antioxidant enzymes, cause oxidative stress and may damage or kill cells.
As oxidative stress might be an important part of many human diseases, the use of antioxidants in pharmacology is
intensively studied, particularly as treatments for stroke and neurodegenerative diseases. However, it is unknown
whether oxidative stress is the cause or the consequence of disease.
Antioxidants are widely used as ingredients in dietary supplements in the hope of maintaining health and preventing
diseases such as cancer, coronary heart disease and even altitude sickness.
Antioxidant
An antioxidant is a molecule capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction
that transfers electrons from a substance to an oxidizing agent. Oxidation reactions can produce free radicals.
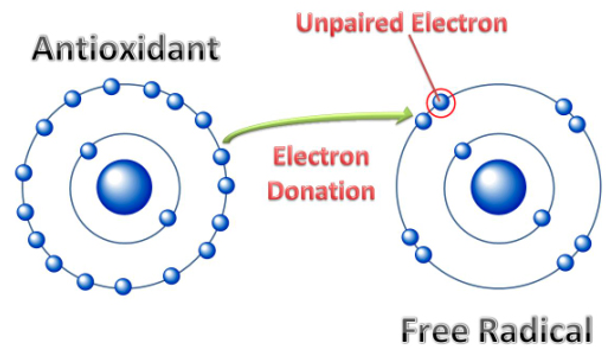
In turn, these radicals can start chain reactions that damage cells. Antioxidants terminate these chain reactions by removing
free radical intermediates, and inhibit other oxidation reactions. They do this by being oxidized themselves, so
antioxidants are often reducing agents such as thiols, ascorbic acid or polyphenols.
Uses
To reduce free radicals, we ingest antioxidant like Vitamin C , fruits ,vegetables ,the main function of antioxidant is to
neutral the unpaired electrons from the cells, as for the chemical reactions is too complicated for us to understand but
one thing is very important, we need antioxidant to maintain our health .Normal fruits have approximate minus ORP
when it just taking out from tree, vitamin C is also have much higher ORP when it is tablet form. Alkaline water also
has higher ORP to reduce free radical,some research suggests that alkaline reduced water is useful in scavenging
free radicals in the laboratory setting.[2] Tests on in vitro lymphocytes suggest that reduced water can prevent hydrogen
peroxide-induced damage to DNA, RNA and certain proteins.
Electrolyzed water has been used by the food industry to sanitize food products; though effective in bacterial solutions,
Acidic electrolyzed water (pH 2.3–2.6) may have use as a seed surface disinfectant or contact bactericide.
References
1. a b c d e f Sies H (1997). “Oxidative stress: oxidants and antioxidants” (PDF). Exp Physiol 82 (2): 291–5.
PMID 9129943.
http://ep.physoc.org/cgi/reprint/82/2/291.pdf.
2. Shirahata, S.; Kabayama, S.; Nakano, M.; Miura, T.; Kusumoto, K.; Gotoh, M.; Hayashi, H.; Otsubo, K.;
Morisawa, S.; Katakura, Y. (1997). “Electrolyzed–Reduced Water Scavenges Active Oxygen Species and
Protects DNA from Oxidative Damage”. Biochemical and Biophysical Research Communications 234 (1):
269–274. doi:10.1006/bbrc.1997.6622. PMID 9169001.
http://linkinghub.elsevier.com/retrieve/pii/S0006291X97966225. Retrieved 2007-12-21.
3. Lee MY, Kim YK, Ryoo KK, Lee YB, Park EJ (2006). “Electrolyzed-reduced water protects against oxidative
damage to DNA, RNA, and protein”. Applied Biochemistry and Biotechnology 135 (2): 133–44. doi:10.
1385/ABAB:135:2:133. PMID 17159237.
4. Abbasi PA, Lazarovits G (October 2006). “Effect of acidic electrolyzed water on the viability of bacterial and
fungal plant pathogens and on bacterial spot disease of tomato”. Canadian journal of microbiology 52 (10): 915–23.
doi:10.1139/w06-048. PMID 17110959.
“Drink the right water, Drink Chanson Water“
