What is pH?
Chemical reaction: pH = 1/log[H+] = -Iog[H+]
pH 6 is ten times more acid than pH 7,pH 5 is a hundred times more acid than pH 7.
On the pH scale, which ranges from 0 on the acidic end to 14 on the alkaline end, a solution is neutral if its pH is 7.
At pH 7, water donations equal concentrations of H+ and OH- ions. Substances with a pH less than 7 are acidic because they contain a higher concentration of H+ ions. Substances with a pH higher than 7 are alkaline because they contain a higher concentration of OH- than H+. The pH scale is a log scale so a change of one pH unit means a tenfold change in the concentration of hydrogen ions.
Pure (neutral) water has a pH around 7; acid or alkali is dissolved in water the pH will be greater than 7 or lower than 7.A solution of a strong acid, such as hydrochloric acid has a pH of 0. A solution of a strong alkali, such as sodium
hydroxide has a pH of 14. Thus, measured pH values will mostly lie in the range 0 to 14.
General symptoms of acidosis, [1] [2] resulting from decrease in body pH.
The pH of different cellular compartments, body fluids, and organs is usually tightly regulated in a process called acid-base homeostasis.
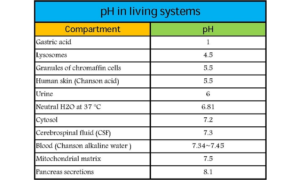
The pH of blood is usually slightly basic with a value of pH 7.4. This value is often referred to as physiological pH in biology and medicine.
Plaque can create a local acidic environment that can result in tooth decay by demineralization.
Enzymes and other proteins have an optimum pH range and can become inactivated or denatured outside this range.
The most common disorder in acid-base homeostasis is acidosis, which means an acid overload in the body, generally defined by pH falling below 7.35.
(Wikipedia)
References
1. Boron, Walter, F.; Boulpaep, E.L. (2004). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders.
ISBN 1-4160-2328-3.
2. Answers.com Medical Encyclopedia: Metabolic Acidosis: Causes and symptoms By Altha Roberts Edgren. Retrieved on April 13, 2009
3. Symptoms mentioned in both metabolic and respiratory acidosis from the following two references: – Wrongdiagnosis.com> Symptoms of Metabolic Acidosis Retrieved on April 13, 2009 – Wrongdiagnosis.com > Symptoms of Respiratory acidosis Retrieved on April 13, 2009
Bad diet or big stress will cause unbalance of pH in your body:
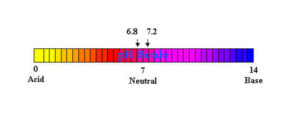
Most interior living matter (excluding the cell nucleus) has a pH of about 6.8. Blood plasma and other fluids that surround the cells in the body have a pH of 7.2 to 7.45.
A blood pH of 6.9 can induce coma and death, Like cancer patient’s saliva is around pH of 4.5~5.7 That is why all bodily systems are secondary in importance to the system of pH balancing.
Through bad diet hobby and stress the body will low down the digestion, alter temperature, rob our bones of calcium, or deprive our pancreas to maintain adequate fluid buffers of alkalinity to balance the acid tide of our body.
At the same time our body has the special mechanism to balance our pH in the body called buffers. What is buffers, main function in our body? bond ions and removes ions to stable the body’s pH, this is very important function because of lost balance will cause disease to our body that is also called bad metabolism for our health.
From above description, enough alkaline water or alkaline foods are very important for our daily life. If ingest junky food with bad hobby like sleep late for every day, disease and quick aging are not far away for both of us.
